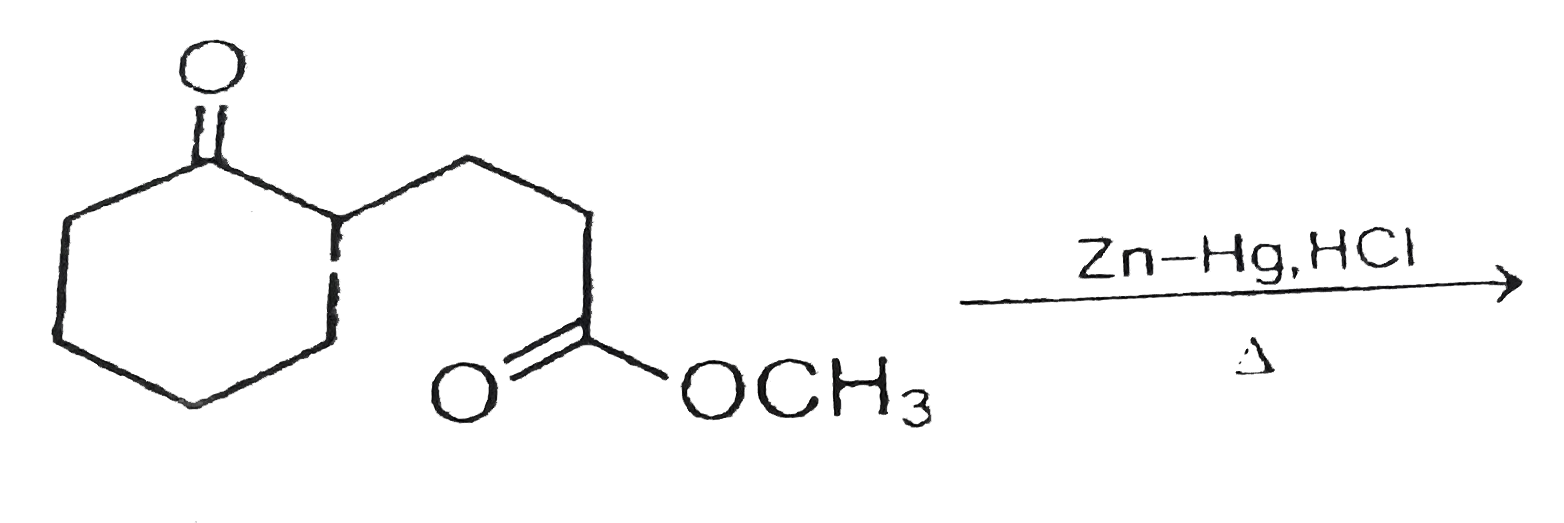
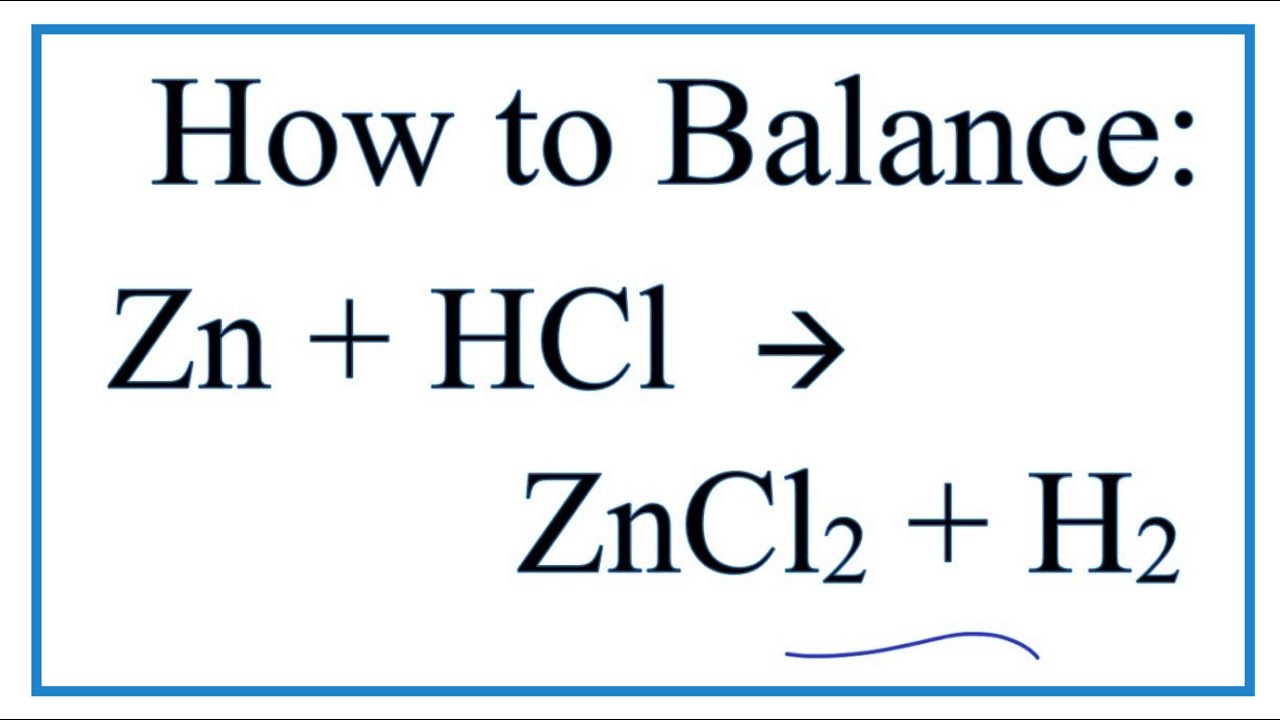
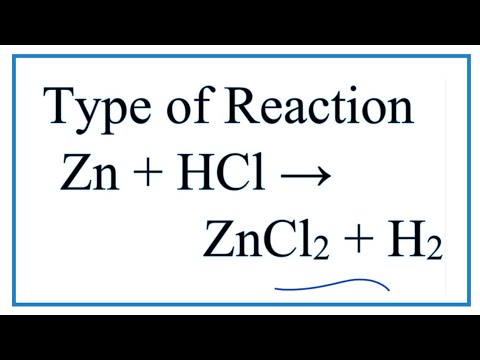
Trimetallic Cu–Ni–Zn/H-ZSM-5 Catalyst for the One-Pot Conversion of Levulinic Acid to High-Yield 1,4-Pentanediol under Mild Conditions in an Aqueous Medium | ACS Catalysis

Hydrosilane σ‐Adduct Intermediates in an Adaptive Zinc‐Catalyzed Cross‐dehydrocoupling of Si−H and O−H Bonds - Patnaik - 2021 - Chemistry – A European Journal - Wiley Online Library
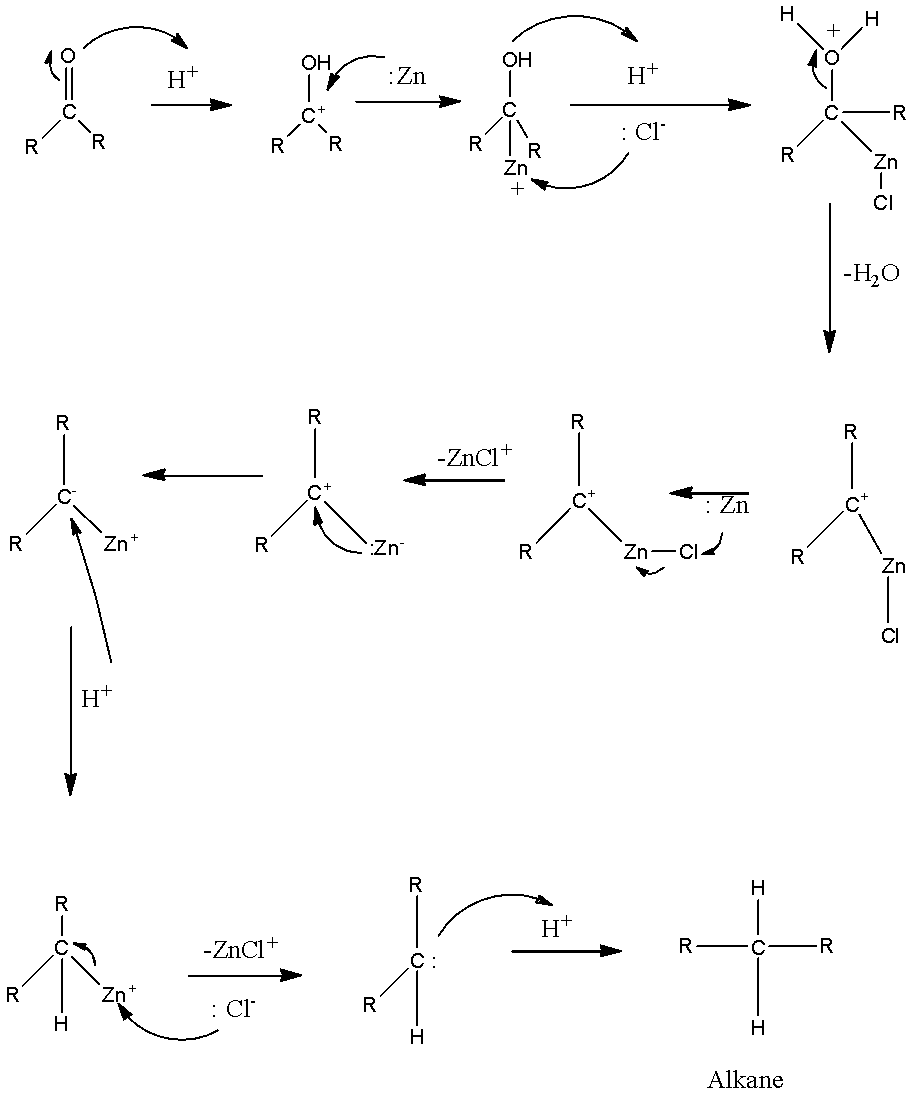
Which of the following is reduced with Zn-Hg and HCl to give alkane?(a).Ethyl acetate(b).Acetic acid(c).Acetamide(d).Butan-2-one


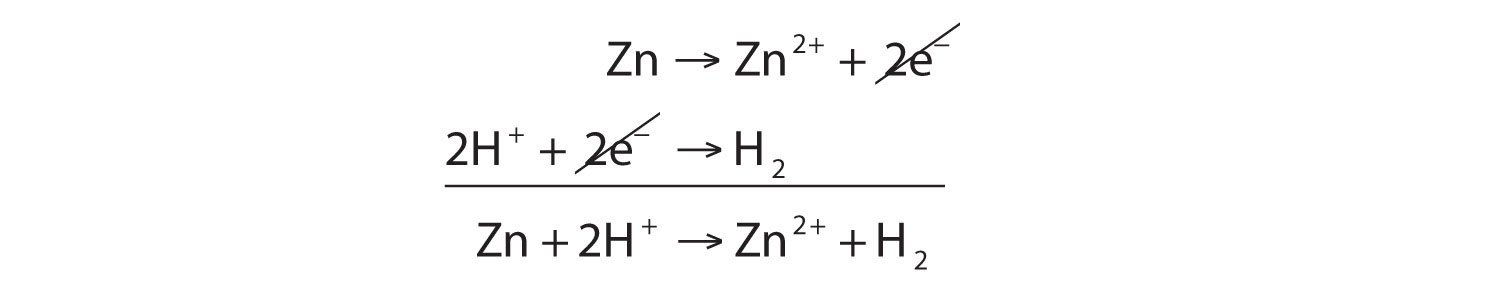

![In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is: In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is:](https://dwes9vv9u0550.cloudfront.net/images/7671796/5bdff3d8-c0c9-4fc8-89cd-f9235a0c1b30.jpg)